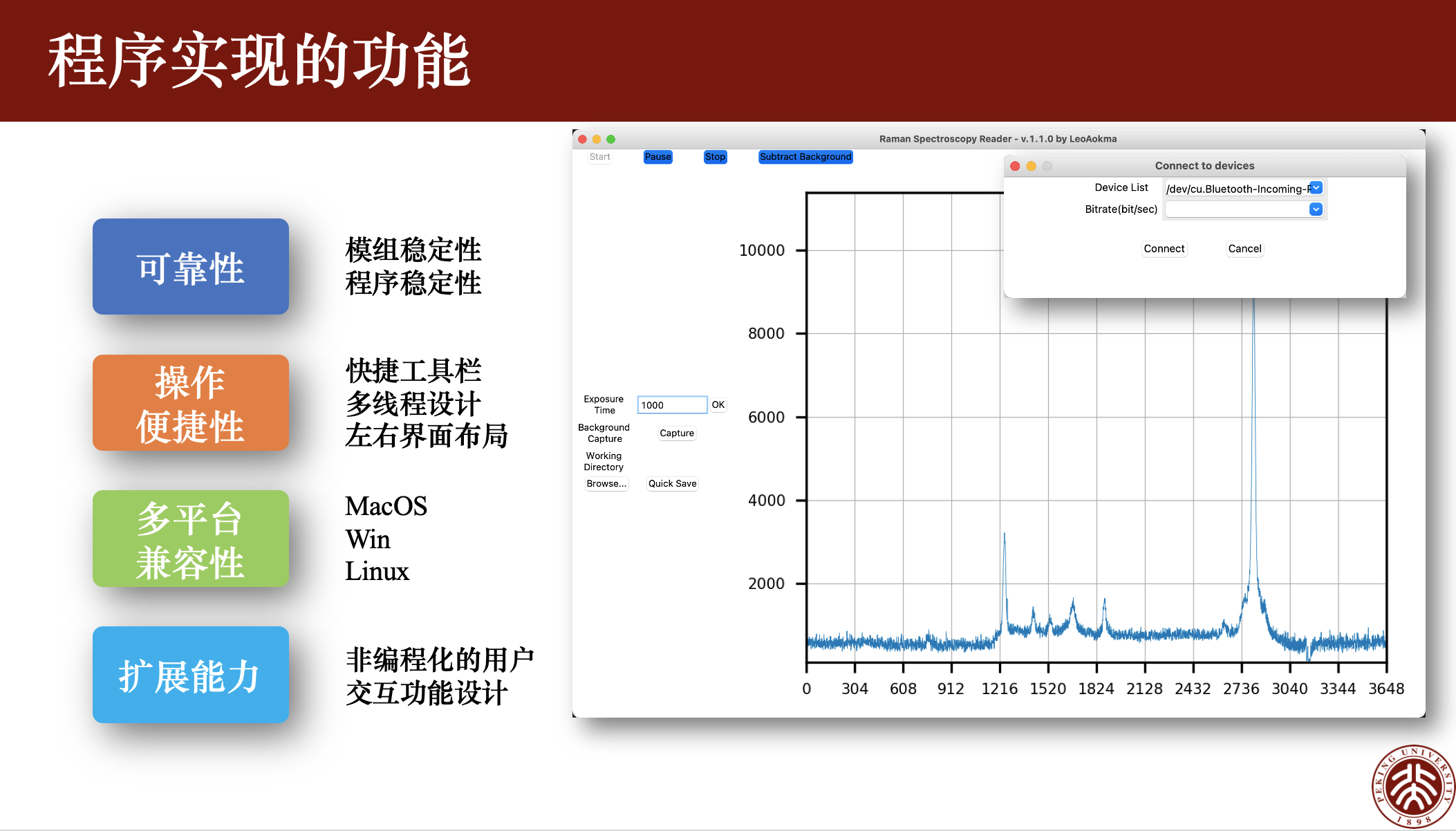
Key Words: Ibuprofen; Synthesis; Iodine Reagents;
Ibuprofen; Synthesis; Iodine Reagents;
Ibuprofen serves as an important molecule in elementary medical treatment. In laboratory synthesis, finding a high-yield, mild-condition and rapid route is of necessity, which provides a guidance to industry production. On the other hand, a quick workup could be used for education purposes. The passage reproduces and develops the synthesis route of Ibuprofen by 1,2-Aryl rearrangement using Iodine reagents, Iodine and (diacetoxy-iodo)benzene particularly. Though reported in quite high yield previously, the reproducibility is critical, resulting from out-of-date papers and lack of experimental data. After an amount of experiments, the experiment process was enhanced and the reaction condition was adjusted to the best yield. The total yield started from precursor 2-Phenylbutane outnumbers 80%. Meanwhile, we characterized and confirmed several co-product obtained in the synthesis, which helped us clarify the mechanism and the selectivity of the reaction.
Ibuprofen; Synthesis; Iodine Reagents;
Ibuprofen serves as an important molecule in elementary medical treatment. In laboratory synthesis, finding a high-yield, mild-condition and rapid route is of necessity, which provides a guidance to industry production. On the other hand, a quick workup could be used for education purposes. The passage reproduces and develops the synthesis route of Ibuprofen by 1,2-Aryl rearrangement using Iodine reagents, Iodine and (diacetoxy-iodo)benzene particularly. Though reported in quite high yield previously, the reproducibility is critical, resulting from out-of-date papers and lack of experimental data. After an amount of experiments, the experiment process was enhanced and the reaction condition was adjusted to the best yield. The total yield started from precursor 2-Phenylbutane outnumbers 80%. Meanwhile, we characterized and confirmed several co-product obtained in the synthesis, which helped us clarify the mechanism and the selectivity of the reaction.
SERS; Raman Spectroscopy;
Ibuprofen serves as an important molecule in elementary medical treatment. In laboratory synthesis, finding a high-yield, mild-condition and rapid route is of necessity, which provides a guidance to industry production. On the other hand, a quick workup could be used for education purposes. The passage reproduces and develops the synthesis route of Ibuprofen by 1,2-Aryl rearrangement using Iodine reagents, Iodine and (diacetoxy-iodo)benzene particularly. Though reported in quite high yield previously, the reproducibility is critical, resulting from out-of-date papers and lack of experimental data. After an amount of experiments, the experiment process was enhanced and the reaction condition was adjusted to the best yield. The total yield started from precursor 2-Phenylbutane outnumbers 80%. Meanwhile, we characterized and confirmed several co-product obtained in the synthesis, which helped us clarify the mechanism and the selectivity of the reaction.